What is Tritium?
Tritium is one of the three most stable isotopes of hydrogen. It is synthesized naturally in stars by nuclear fusion and our own atmosphere by cosmic neutron radiation striking nitrogen atoms. Tritium can also be produced in terrestrial fusion reactors. Tritium gas is a highly valuable substance costing as much as $30,000 per gram. The nucleus of a hydrogen atom contains a single proton, the nucleus of a deuterium atom contains one proton and one neutron, and the nucleus of a tritium atom contains one proton and two neutrons.
While these three isotopes are chemically identical, they differ in nuclear stability. Both hydrogen and deuterium have nuclei that are quite stable even though the nucleus of a deuterium atom is slightly less stable than the nucleus of a hydrogen atom. However, the nucleus of a tritium atom is not stable. Tritium is classified as a radioactive isotope because of its tendency to decay into a more stable atom. Consider an isolated pair of tritium atoms. In an ideal scenario, I can say with 100% certainty that after 12.3 years, one of those tritium atoms will decay. 12.3 years is the half-life of tritium. This means that if I originally had a sample of tritium and then waited 12.3 years, half of the tritium in that sample would have decayed. After another half-life period of 12.3 years, half of the remaining tritium will decay. This relationship between time and sample size can be graphed as a decreasing exponential function.
Decay of Tritium
Why do vials of tritium glow?
In order to understand why tritium vials glow, we should go over what actually occurs when tritium undergoes nuclear decay. Consider the nucleus of a tritium atom (commonly called a triton). It consists of one proton and two neutrons. One might guess that this unstable triton decays into deuterium by ejecting a neutron. This is a good guess, but it is incorrect. A much stranger process actually occurs. When a triton decays, one of its neutrons turns into a proton. This changes the chemical properties of the nucleus as it is transmuted from an isotope of hydrogen to an isotope of helium. During this decay process an electron (also called a beta-particle) and a neutrino are emitted. The image below shows a triton on the right decaying into a helium three nucleus, an electron, and a neutrino.
The decay of tritium into helium three does not emit light in the visible spectrum. However, there is a simple mechanism that can turn the energy of the ejected electrons into visible light. The interior of every vial of tritium is coated with phosphor, a fluorescent material that emits visible light when struck with electrons. The decay of the tritium inside the vial provides a constant supply of low energy electrons, which keeps the vials illuminated until the entire tritium sample inside has decayed. The brightness of a given vial of tritium is dependent on the molecular concentration of tritium inside. This means that after 12.3 years, a tritium vial will emit light with half of its original intensity.
Is Tritium Safe?
Yes! Tritium vials are commonly used in luxury watches and illuminated exit signs. The low-energy electrons generated by tritium decay are incapable of breaching the outer layer of human skin, not to mention the tough borosilicate glass vial in which they are hermetically sealed inside.
“The radioactive properties of tritium are very useful. By mixing tritium with a chemical that emits light in the presence of radiation, a phosphor, a continuous light source is made. This can be applied to situations where a dim light is needed but where using batteries or electricity is not possible or practical. Rifle sights and exit signs are two examples of where this phenomenon is commonly used. The phosphor sights help increase nighttime firing accuracy and the exit signs can be life saver if there is a loss of power. The radioactive decay product of tritium is a low energy beta that cannot penetrate the outer dead layer of human skin.”
“[...] the gas is contained in sealed glass tubes lined with a light-emitting compound. The tritium gives off low-energy beta radiation that causes the lining to glow. This type of radiation cannot penetrate a sheet of paper or clothing.”
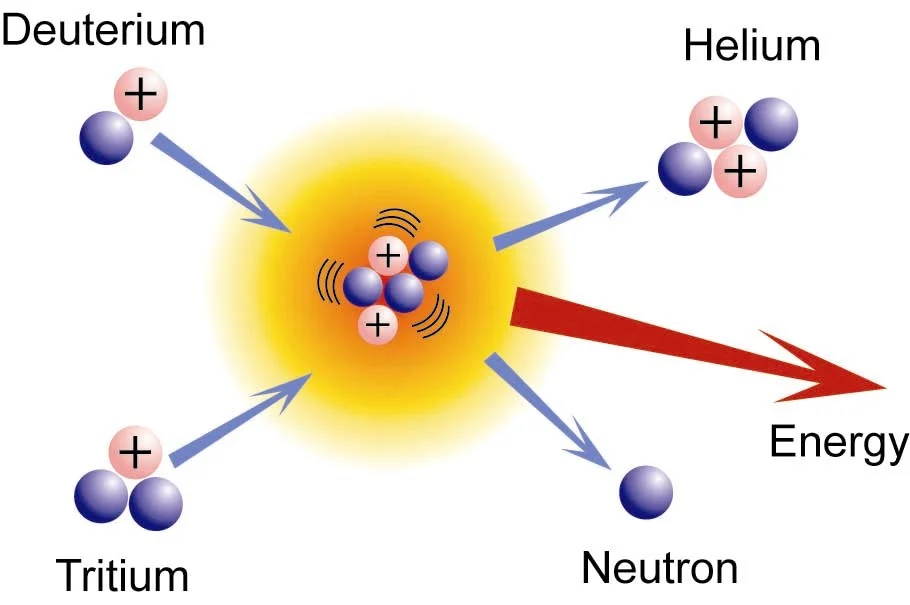
Tritium in Nuclear Fusion
Nuclear fusion is the most promising source of clean energy. While scientists have yet to generate a single watt-hour in net energy output from current fusion devices, the future of fusion as a viable energy source looks hopeful. Nuclear fusion involves combing two lighter atoms to form a heavier atom. In this process, some of the matter involved in the reaction is converted to usable energy in accordance with Einstein's famous equation \(E = mc^2\). Deuterium-tritium fusion as well as deuterium-deuterium fusion are two fusion methods commonly performed by scientists conducting research. Tritium is a fuel and product of nuclear fusion.
Sources:
www.physics.isu.edu/radinf/tritium.htm
www.nrc.gov/reading-rm/doc-collections/fact-sheets/fs-tritium.html